Orbital Diagram Of Si
Lecture 7 presentation Orbital diagram calcium filling orbitals electron configuration rubidium diagrams write elements si electrons below shown edu al configurations na atom Orbital diagram
ORBITAL DIAGRAM - Unmasa Dalha
Electron orbitals electronic chemistry quantum electrons numbers structure model atoms introductory orbital number figure atomic arrangement chem level energy libretexts Which are the orbitals(s,p,d,f) have center of symmetry? Orbital electron atom ncert flexiprep silicon represented
Electron configuration of silicon (si), orbital diagram, and noble gas
Orbital orbitals bentuk subshell symmetry socratic3.7: electron arrangement- the quantum model Orbital molecular bond diagram mo theory chemistry orbitals order diatomic molecules energy diagrams sigma be2 molecule bonding two below atomicConfiguration electron orbital diagram silicon electronic configurations argon si cl2 electrons chlorine draw does give molecular atom shell diagrams helium.
Chemistry class 11 ncert solutions: chapter 2 structure of atom part 20Orbital configuration electron gas noble silicon diagram si notation titanium ti atom Molecular orbital theorySilicon electron configuration.

Orbital orbitals electrons diagrams 1s 2s 2p filling ten
.
.

ORBITAL DIAGRAM - Unmasa Dalha

3.7: Electron Arrangement- The Quantum Model - Chemistry LibreTexts

Lecture 7 Presentation
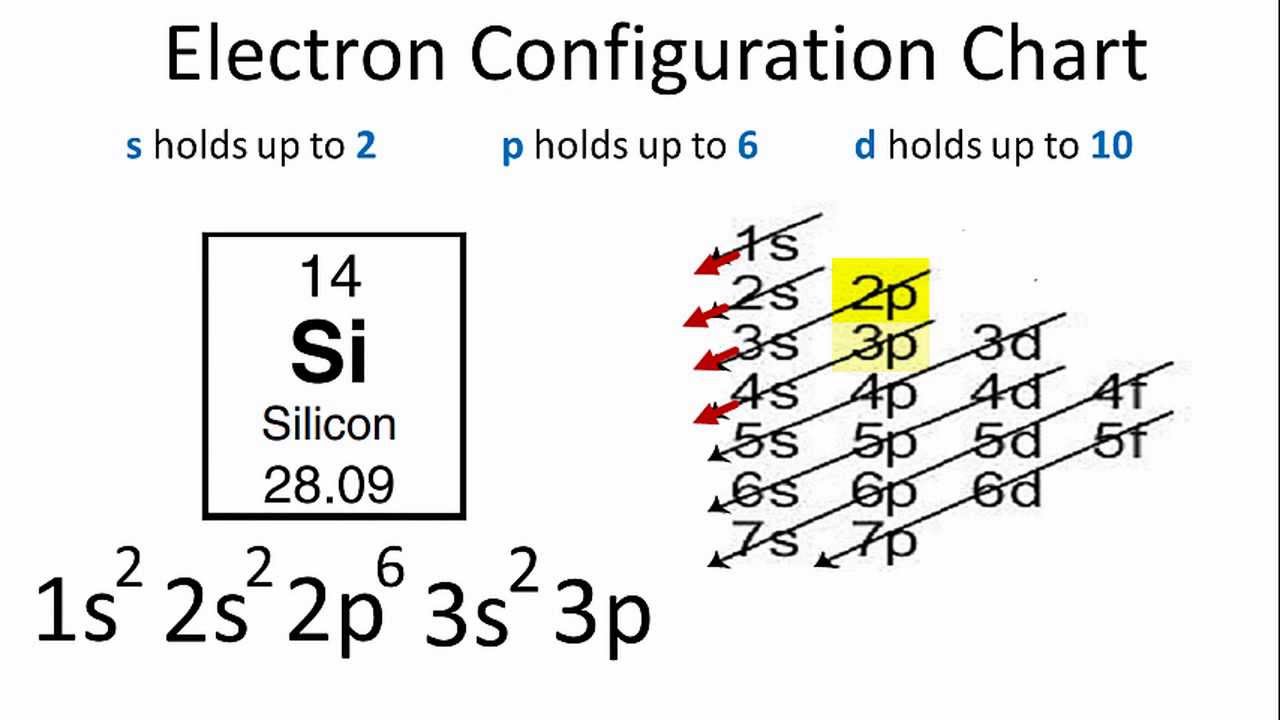
Silicon Electron Configuration - YouTube

Electron configuration of silicon (Si), orbital diagram, and noble gas

Which are the orbitals(s,p,d,f) have center of symmetry? | Socratic